
INTRODUCTION
In the realm of medical advancements, one area that has long posed a challenge is delivering effective treatments to the central nervous system (CNS). Neurodegenerative diseases, malignant conditions, and CNS injuries can be devastating, and their treatment often necessitates innovative solutions. Enter the Porous Brain Infusion Catheter (PBIC), a game-changing medical device created by OCCAM Design that promises to revolutionize drug delivery to the brain.
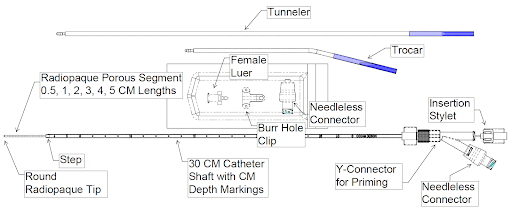

THE CHALLENGE: DELIVERING THERAPIES TO THE CNS
Neurological diseases and injuries affecting the central nervous system pose immense difficulties for treatment. The protective barrier known as the blood-brain barrier makes it challenging for drugs to reach their intended targets in the brain. For effective treatment, direct delivery to lesions in the CNS is often required.
Convection-enhanced delivery (CED) has emerged as a promising approach to bypass the blood-brain barrier. It enables the infusion of drugs directly into the brain, allowing for precise and targeted therapy delivery. However, even CED faces its own set of challenges, particularly when large regions of the brain need to be treated. Poor drug distribution within the brain tissue has been a significant hurdle in realizing the full potential of CED.
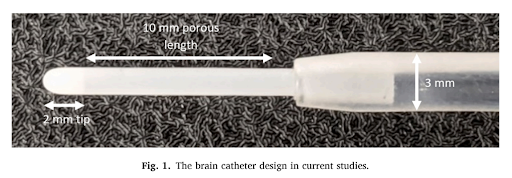

THE SOLUTION: THE POROUS BRAIN INFUSION CATHETER
As a result, OCCAM Design has taken on this challenge and developed the Porous Brain Infusion Catheter (PBIC). Compared to standard catheters, this innovative catheter design promises a more uniform and efficient distribution of infusates within the CNS tissue. Improved distribution holds the potential to significantly enhance the effectiveness of drugs and other therapies delivered to the brain. The development of this catheter could lead to greater efficiency in administering medications to patients, which allows for enhanced healthcare.
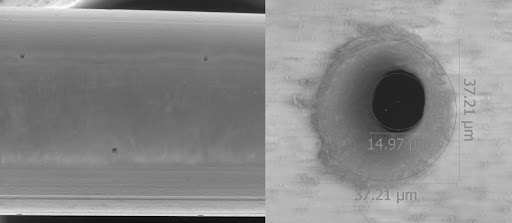

ACCOMPLISHMENTS: A JOURNEY OF PROGRESS
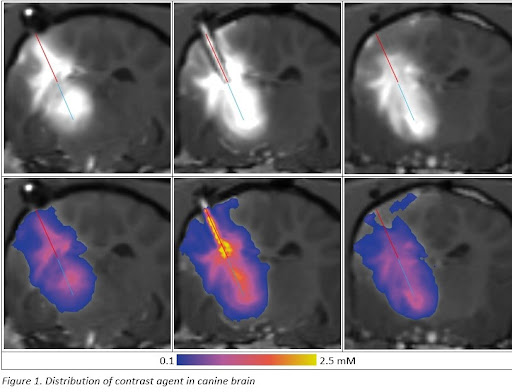
The PBIC project has achieved notable milestones that signal a promising future for neurological drug delivery. First, the PBIC underwent rigorous safety testing, paving the way for its submission to the Food and Drug Administration (FDA). Next, plans for conducting human trials with Duke University are in progress. Our partnership with Duke University for human trials underscores the project’s dedication to advancing treatment options. Our goal is to finish and submit a device master file with the FDA. Preparing a device master file with the FDA demonstrates OCCAM Design’s commitment to meeting regulatory standards. Most recently, our team is in the process of conducting chronic canine model testing. Ongoing tests in a chronic canine model at the University of Kentucky will prove the safety and efficacy of the PBIC over 90 days. In addition, we are honored to have publications in the “Journal of Neuroscience Methods” based on the creation of the Porous Brain Infusion Catheter. Preliminary studies have been published in the Journal of Neuroscience Methods, highlighting the innovative approach and its potential impact. One of the most exciting impacts of this medical device development is the opportunity for future developments for spinal tumors. The porous design, initially developed for brain infusions, is now being further developed to treat spinal tumors, expanding its potential applications.

The Porous Brain Infusion Catheter stands at the forefront of neurological drug delivery innovation. Its potential to improve drug distribution within the CNS tissue brings hope to patients with devastating neurological conditions. As OCCAM Design continues to push the boundaries of medical technology, the future promises more effective treatments and better outcomes for individuals battling CNS diseases and injuries.
Efficiency and new and improved medical devices are at your fingertips. Partner with OCCAM Design to develop, manufacture, and distribute medical devices like the Porous Brain Infusion Catheter that could be life-changing for your company and your patients.
