
Technology continues to advance in all facets of the medical field and healthcare. Medical device software development has become integral to the digital platforms serving the healthcare and non-healthcare industries. This software development has been a game-changer for the industry.
Medical device software comes in 4 primary subclasses:
- Software as a medical device (SaMD), which is a standalone software that serves as a medical product in and of itself;
- Software in a medical device (SiMD). This is software that’s part of a medical product, such as implanted software in medical equipment;
- Software as an accessory to a medical device;
- General purpose software that is not a medical product by itself.
What is Software in a Medical Device?
Any software that helps to run a hardware medical device— by powering its mechanics or producing a graphical interface—is software in a medical device. Some examples include
- Software that controls the inflation or deflation of a blood pressure cuff
- Software that controls the delivery of insulin on an insulin pump
- Software used in a closed loop control of a pacemaker

Examples of SiMD
Types of software that receives information from an MRI machine and converts it to images. The software that analyzes the images would be a SaMD.
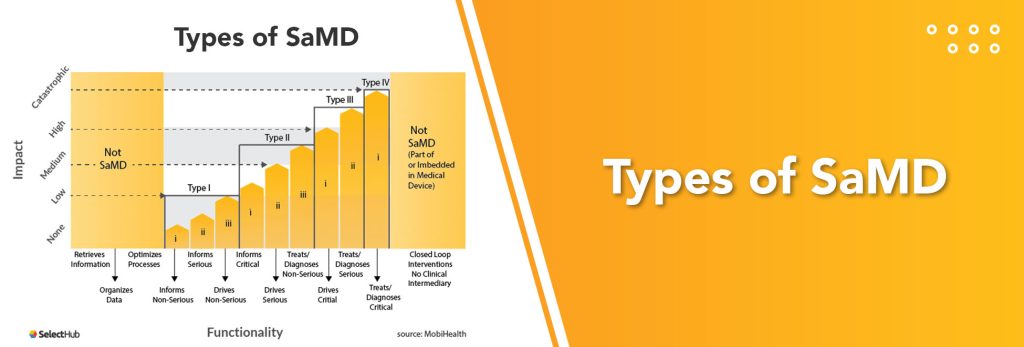
What is Software as a Medical Device?
Software as a Medical Device (SaMD) is defined by the International Medical Device Regulators Forum (IMDRF) as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.” This would include software or mobile applications intended to treat, diagnose, cure, mitigate, or prevent disease or other conditions.
Software as a Medical Device has become more prominent over the last few years. According to the Federal Drug Administration (FDA), it can be used across a broad range of technology platforms, including medical device platforms, commercial “off-the-shelf” platforms, and virtual networks, to name a few. Such software was previously referred to by industry, international regulators, and healthcare providers as “standalone software,” “medical device software,” and/or “health software,” and can sometimes be confused with other types of software.

For any software to be classified as SaMD, it must stand alone from any hardware as it performs the functions that categorize it as a medical device. The International Medical Device Regulators Forum clarifies SaMD as the following:
- SaMD is a medical device and includes in-vitro diagnostic (IVD) medical devices.
- SaMD is capable of running on general purpose (non-medical purpose) computing platforms.
- “Without being part of” means the software is not necessary for a hardware medical device to achieve its intended medical purpose.
- Software does not meet the definition of SaMD if its intended purpose is to drive a hardware medical device.
- SaMD may be used in combination (e.g., as a module) with other products, including medical devices.
- SaMD may be interfaced with other medical devices, including hardware medical devices, other SaMD software, and general purpose software.
- Mobile apps that meet the definition above are considered SaMD.
Examples of SaMD
Patient Data: Software that collects patient data like blood pressure, heart rate, and weight. This provides real-time data to medical professionals monitoring remotely.
Image Data: Software used to analyze and manipulate images collected from radiation-emitting imaging devices that create 3D models. This software assists with treatment plans and prevents invasive exploratory surgery and exposure to radiation through x-rays, CT scans, and MRIs. This is used in orthopedics and dental surgical treatments.
Treatment Data: This software uses algorithms to analyze patient information to determine a treatment plan. The data required for the algorithm include patient weight, age, blood pressure, and heart rate. The software would collect additional patient information for input depending on the type of treatment required. The medical professional requires this type of software to decipher the data made by the algorithm.
Sleep Data: This software analyzes physiological signals previously collected from sleep lab settings. It determines sleep stages, takes snoring measurements, detects arousals, and assists in assessing sleep quality. The data can be analyzed and used for treatment plans. This software can be used to identify obstructive sleep apnea.
Ear Exam: Utilizing a smartphone camera and an otoscope attachment, this software can be used to perform an ear self-exam. It captures video of the inside of the ear, which is then used to support a diagnosis. This particular software launches the camera, controls when the images are collected, and performs storage and sharing functions.
Breast Screening: This software calculates breast density percentage using the same type of digital mammograms radiologists use. Mammograms are essential to assess breast tissue composition. This software provides an evaluation of the risk of breast cancer in an individual based on the analysis of digital images. The software is key to treatment plans and further testing.
Benefits of a SaMD
The purpose of SaMD is to assist in discovering, managing, and treating medical issues. The software helps to automate aspects of these areas, which can save time and provide more accuracy, contributing to improved patient health outcomes.
REGULATION
There are several markets around the world for medical devices. Two of the largest are the US and the EU. In the US, you must follow the Quality System Regulations (QSR) from the FDA. In the EU, you would follow the guidelines of the ERU MDR (EU IVDR if in vitro device). SaMD requires you to follow the same guidelines as any other medical device.
OCCAM Design’s expert engineers, technicians, and machinists, coupled with comprehensive manufacturing capabilities, provide innovative and reliable solutions to the most challenging applications. Our services include design, development, and manufacturing.

